
Consulting
Detecting duplicated and ineligible chargeback requests in the pharmaceutical industry.
Prepared by Dr. Alexander Werner. KB# 10002.
This case study relied upon the Pharma Suite chargeback processing module, developed by Relasoft Solutions Inc for pharmaceutical manufacturers and distributors.
1. Introduction
The issue of duplicated submissions is one of the well-known problems of chargeback processing in the pharmaceutical industry.
The two principal sources for such duplicates are:
- Unauthorized products submission. Unauthorized product submissions happen when wholesalers fail to report negative chargebacks for product returns, while they do issue second chargeback requests upon re-sell of those products.Unauthorized products also include drugs purchased from third channels and short-dated drugs purchased at discount.
- Duplicated credit requests for the same sale. Wholesalers submit duplicated chargeback requests for the same transaction more than once. The requests are considered duplicates when they match by NDC, wholesaler invoice number and invoice date, pharmacy DEA and the number of units sold.
Let’s review both sources and see which aspects of validation can be implemented by the pharmaceutical chargeback processing software.
2. Unauthorized Product Submissions
The vast majority of chargeback requests – 99.67% in the pharmaceutical industry – are coming via EDI in the format of the form 844. Neither this form, nor standard paper-based submission form, provide any identification details about the drugs sold, other than their NDC numbers. The absence of unique item identifier makes it impossible to determine item’s eligibility for the chargeback based on the information contained in the request.
The only alternative route of detecting unauthorized products is to rely on the validation by inventory, considering that only one suitable error code supported by EDI is A1 – Insufficient Wholesaler Inventory. The other similar code A3 – Quantity Invalid – Free Goods – is impossible to prove and apply if the wholesaler purchased some drugs and received other drugs with the same NDC as promotions.
This algorithm of validation by inventory combines sales and chargeback data with wholesalers’ at-hand inventory counts – with the information often coming from various computer systems and in different formats – and calculates the maximum quantity of units permitted to receive chargebacks per wholesaler for each NDC, as illustrated on the diagram below:
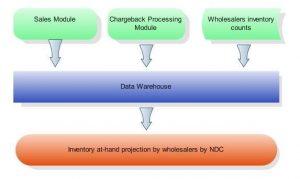
Duplicated Chargback in Pharmaceutical Industry
The calculations have to take into account the following factors, contributing uncertainties into the processing:
- Drugs may be transferred between wholesalers’ distribution centers, meaning that “negative” projected inventory at hand at a certain distribution center does not constitute a reason to reject chargebacks coming from that distribution center;
- Chargeback submissions may be delayed for weeks, months, and even years. As the result, chargeback for a later sale may be approved while the chargeback for the prior one rejected. Tejected chargeback requests may be re-submitted at a later date;
- Wholesalers have non-contractual sales diminishing their inventory, but not reflected in the chargebacks submissions. The ratio of non-contractual sales to all sales varies by NDC, wholesalers and a time range.
Pharma Suite marked over 10% of chargeback requests as having inventory balance discrepancies for some NDC and wholesalers. However, either due to the sufficient explanations received from wholesalers or because of lack of proof, many of those transactions in question were approved.
It is interesting to note, that merging sales and chargeback data and obtaining correlated ratios comes with unexpected benefits: those ratios can be used in chargeback smoothing methods in AMP calculations, thus resolving another known issue – reporting overly low or overly high AMP for MDRI program for CMS’s Center for Medicaid.
Combining sales, chargeback and inventory at-hand data helps to detect potential issues and to enforce validation chargeback submission by inventory in semi-automated mode.
3. Duplicated credit requests for the same sale.
Duplicates of the second type occur when either the whole debit memo is repeatedly submitted in separate EDI files, or when the same invoice line is submitted a number of times in different debit memos. The standards of processing EDI forms 844 provide code YY – Duplicated Line Entries – to reject such debit memos or lines, and they can be proven to be duplicates based on the transaction history maintained by the manufacturers or distributors.
Data analysis conducted on the transaction pool of about 30 million records collected over five years, showed that duplicated submissions of the second type constitute 3.67% of all submitted debit memo lines!
The vast majority of the duplicates – 95.8% – happen because the whole debit memos were submitted multiple times, often because of EDI transmission errors. And the good news is that such duplicates are quite easy to notice and handle.
The other 4.2% happen because of repetitive submissions of the individual lines. Out of those records, 44.7% duplicated lines were coming in the same debit memos with the original submission, while the other 55.3% duplicated lines were submitted a number of days, months and, yes, even years after submissions of the original lines!
Even with monthly volume of chargebacks measured in hundreds of thousands and millions transactions, getting computer systems with enough computational power to provide such validation does not present a challenge nowadays.
Any professional pharmaceutical chargeback processing software implementing the five point check described above will automatically prevent overpayments due to the duplicated credit requests for the same sale.
4. Conclusion
Duplicated pharmaceutical chargebacks include unauthorized products submission and duplicated credit requests for the same sale. Tight software integration between sales, chargebacks and wholesalers inventory counts is required to validate submissions by the inventory in semi-automated mode. Professional software is required to automatically reject duplicated requests for the same transactions.
Alexander Werner, Ph.D, is a founding partner and the chief software architect for Relasoft Solutions Inc, the company specializing in software for Healthcare Management industry. Relasoft main product Pharma Suite includes modules to process several typical tasks in the pharmaceutical environment: Chargebacks, Rebates, MDRI and Coverage Gap Discount program for Medicaid, Sales Commission, Business Intelligence and reporting systems.
Here are the links for more info on Pharma Suite and to contact Alexander.
